
The best way to understand flowcharts is to look at some examples of flowcharts. Audit a process for inefficiencies or malfunctions.Program or system design through flowchart programming climate change, global climate change, global warming, natural hazards, Earth, environment, remote sensing, atmosphere, land processes, oceans, volcanoes, land cover.Here are some of the ways flowcharts are used today. Today, flowcharts are used for a variety of purposes in manufacturing, architecture, engineering, business, technology, education, science, medicine, government, administration and many other disciplines. Work processes such as assembly line manufacturing. Here are just a few ofįlowcharts were originally used by industrial engineers to structure There are a wide variety of flowchart types. Today's flowcharts are typically created using a flowchart maker.

Customers can also order most of these data as certified hard copies for legal use. Helped flowchart makers work more quickly and gave their diagrams These data include quality controlled daily, monthly, seasonal, and yearly measurements of temperature, precipitation, wind, and degree days as well as radar data and 30-year Climate Normals.
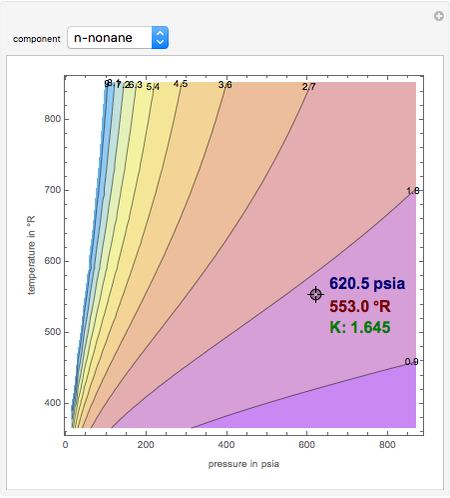
Originally, flowcharts were created by hand using pencil and paper.īefore the advent of the personal computer, drawing templates made of plastic flowchart shape outlines There are several ways to make a flowchart. Read our complete guide to flowchart symbols. Usage conveys a certain message, flowchart symbols also have specific meaning. Important to know what they represent before using them. These shapes are known as flowchart symbols. Points of the process and rectangles are used to show the interim steps. There are two shapes: those with rounded ends represent the start and end Thermodynamic calculations using K-values Dew point Procedure: a) Select T b) Ki(T) c)Ĭampuran terdiri n-hexane (0.15), n-heptane (0.20), n-octane (0.30) dan n-nonane (0.You'll notice that the flowchart has different shapes. Y A / K A (T ) + y B / K B (T ) = 1 - Thus as we decrease the temperature we put new K-values the above equation until this condition is met Y A + y B = 1 - Consider the process in the figure: we start with a mixture of composition 1 and temperature T1 and start decreasing the temperature - As we decrease the temperature we are going to reach a point where the first drop of liquid forms - The liquid in the droplet obeys: Thermodynamic calculations using K-values Dew point Thermodynamic calculations using K-values Bubble point Procedure: a) Select T b) Ki(T) c)Į) Adjusting T g) Final composition can be corrected usingĬampuran pada tekanan 300 kPa terdiri dari: 50 kmol Heptane 30 kmol Octane 20 kmol Nonane Tentukan bubble point! Gunakan de Priester Chart. K A (T ) x A + K B (T ) x B = 1 - Thus as we increase the temperature we put new K-values in the above equation until this condition is met As we increase the temperature we are going to reach a point where the first bubble forms - The vapour in this bubble obeys: X A + x B = 1 - Consider the process in the figure: we start with a mixture of composition 1 and temperature T1 and start increasing the temperature Thermodynamic calculations using K-values Bubble point - Model system: binary mixture A, B X* Easy for 2 component system, if T-x-y diagram is available (remember the lever rule?) What about the multi-component system? Thermodynamic calculations using K-values P Grafik menunjukkan hubungan nilai K untuk suatu komponen dengan suhu dan tekananĬampuran terdiri n-hexane, n-heptane, noctane dan n-nonane pada suhu 40C dan 500 kPaīubble point is the condition that the first bubble formed during liquid mixture boiled Dew point is the condition that the first drop formed during vapor mixture cooled Persamaan Antoine dan koefisien dinyatakan dalam beberapa bentuk:ĭata koefisien Antoine dapat dilihat di Chemical Engineering Vol 6 Persamaan Antoine memberikan hubungan tekanan uap murni (P atau VP) suatu senyawa/komponen dengan suhu. K i = Pi / P Relative volatility for ideal gas/ideal solution system: s Pi = yi P K-value for ideal gas/ideal solution system: s Pi is the partial pressure of component i Thermodynamic data for mixtures: Simplified models V Ki = yi/xi correlated empirically or theoretically in terms of temperature pressure and composition The ratio of two K-values, or relative volatility, indicates the relative ease or difficulty of separating components i and j Thermodynamic considerations and phase equilibria: multicomponent mixtures For multicomponent mixtures simple graphical representations of vapour-liquid equilibria data do not exist Most often such data (including binary systems) is represented in terms of K values defined as: Penya enyajian jian Data Data Keset Kesetimbang imbangan an (presenting (presenting equilibrium data)


 0 kommentar(er)
0 kommentar(er)
